Nanoscale materials, much in the news in the past decade, are actually not as novel as they are touted to be. What is new is our capacity to synthesize them in almost any size and shape of interest, together with the development of analytical techniques better suited than those available in the past.
Nanoscale materials distinguish themselves from bulk-sized materials by their length scale, usually set at an arbitrary upper limit 100 A. This decrease in particle size (often accompanied by a change in shape) impacts on their physical properties in many different ways, a few of which are mentionned to iillustrate the effect:
a) Increase in the surface-to-volume ratio, potentially useful in the field of catalysis,
b) In semiconductors the scale of the electronic motion becomes smaller than the length scale in bulk material,
c) In noble metals such as gold a new strong absorption is observed, giving the material a brilliant color (pink in the case of gold). This effect has been used by the Chinese to color vases and by medieval European craftsmen to stain glass windows in cathedrals. Physically, this results from the collective oscillation of the free conduction electrons in the conduction bands from one surface of the particles to the other. Its frequency is such that it absorbs visible light.
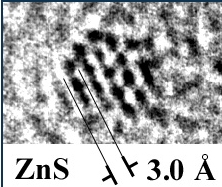
IIn most of the potential industrial application of nanosized materials, the structure and the quality of their surface will play the central role in determining their functions. This is why we have concentrated at first on adapting analytical techniques to the problem at hand