Zeolites are amongst the most useful compound prototypes Nature has given us. Detailing all their manifold uses could easily fill several pages. Let us instead solely mention their three principal areas of industrial application: sorption, ion exchange and catalysis. To fulfill these tasks these microporous (or nanostructured, to use a more timely lingo!) solids compound several winning assets: They are thermally stable, easily separable from the reaction products and conveniently regeneratable.
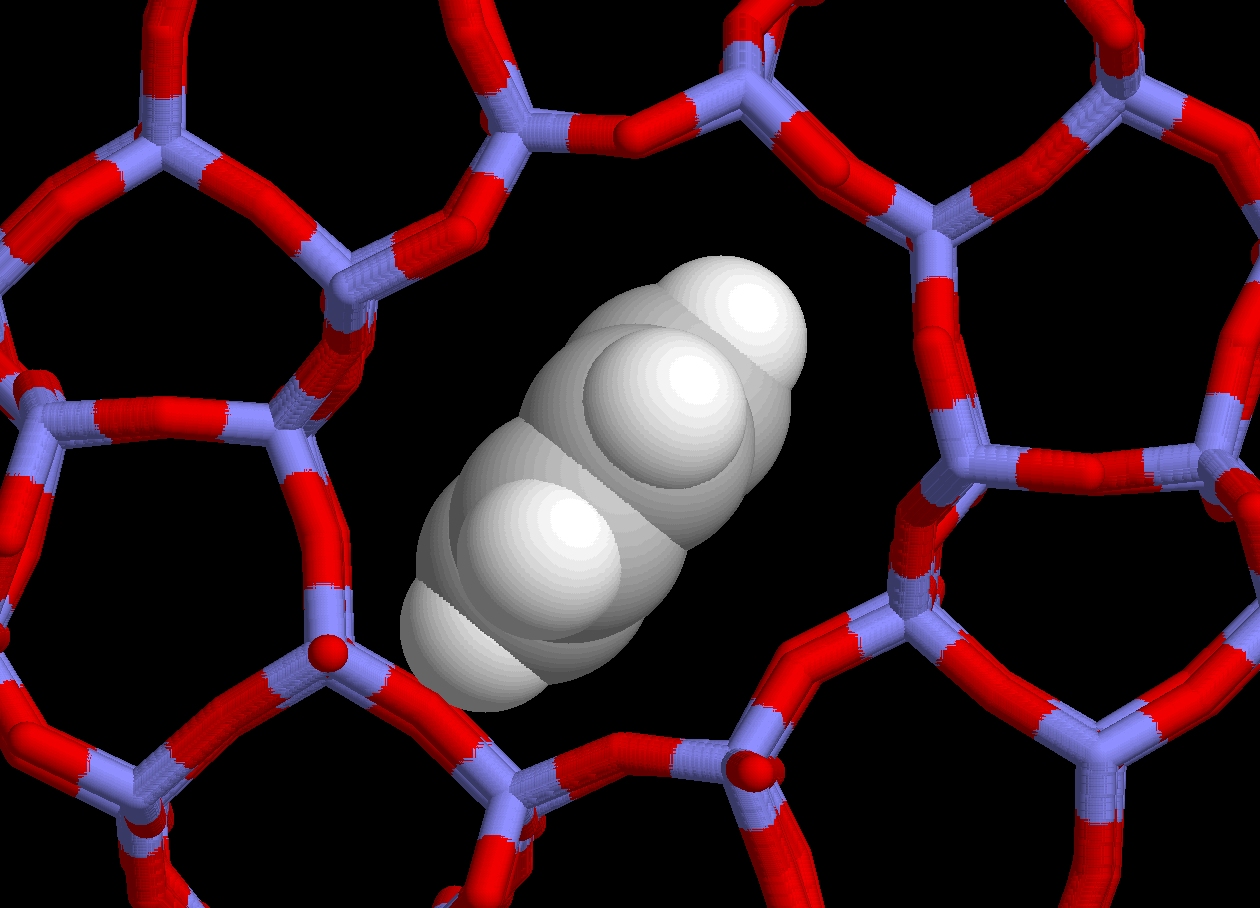
The complexity of the catalytic processes taking place in zeolite is such that it is far too optimistic to expect their full characterization solely from experimental observations; computer simulation is needed to fill in the observational gaps (however it should not substitute itself for a feasible experiment!). If such modeling is not based on sound and complete structural data, it will yield misleading insights. This is why, amongst other things, we aim at providing accurate benchmark structures for subsequent theoretical modeling. Our work aims presently at accurately locating extraframework cations and guest molecules in two of the industrially most relevant zeolites (ZSM5 and faujasite), and this at conditions close to those prevailing when in use.
In the near future we plan on carrying out charge density studies on these and similar materials. We expect them to provide us with information not obtainable by any other experimental technique such as
(HPW, Karl Seff, BFM)